Salts and Acids Are Examples of Inorganic Compounds Called
Ammonium chloride sodium carbonate potassium iodide. Water is an example of a compound.
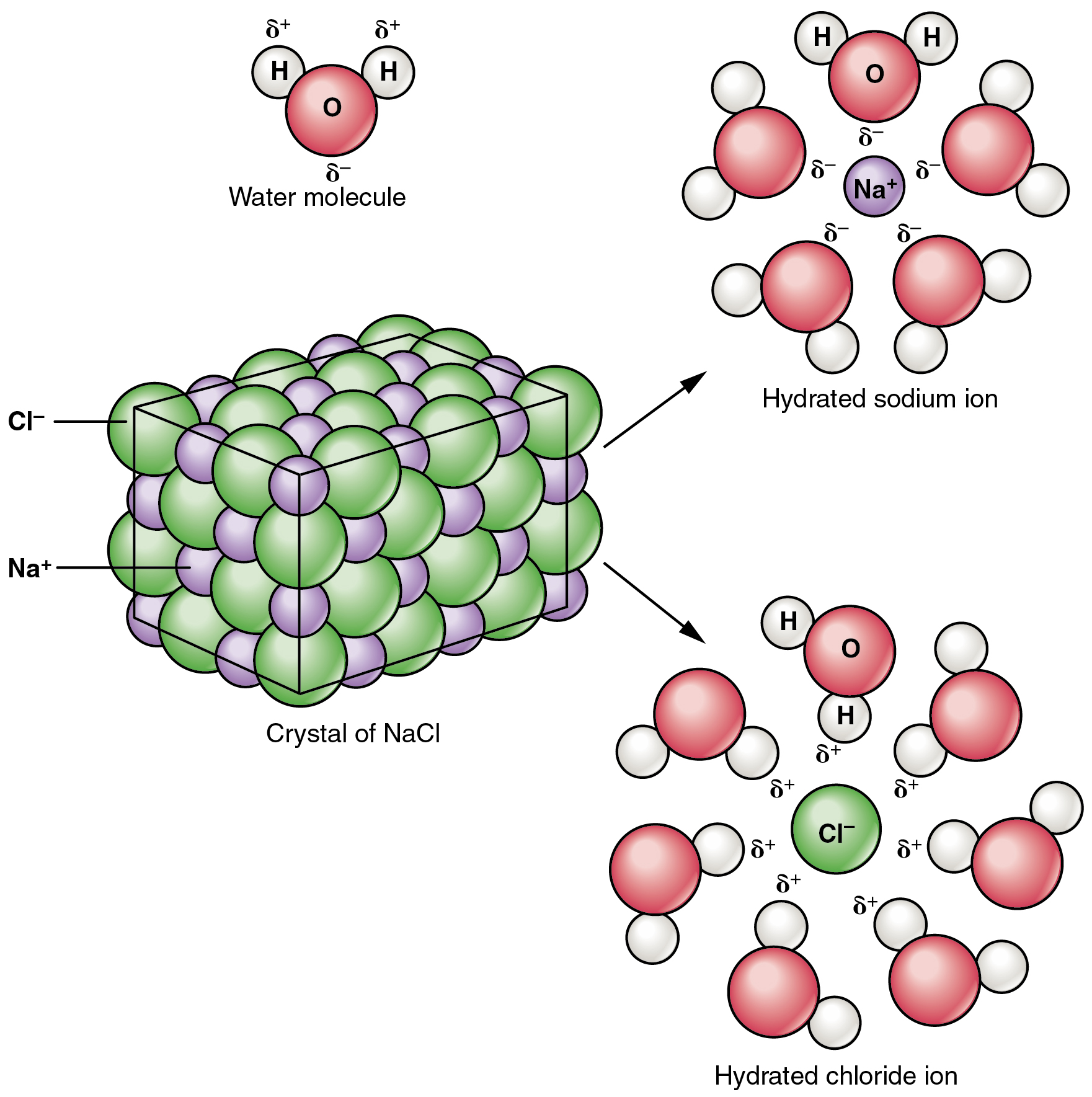
Inorganic Compounds Essential To Human Functioning Anatomy And Physiology
Inorganic acids also called mineral acids are acids derived from one or more inorganic compounds.
. Sulphuric Acid H2SO4 Acids also belong to inorganic compounds. A compound A compound is a substances that contains two or more different atoms that are bonded together. Hydrochloric acid hydrofluoric acid which are monobasic and hydrogen.
In contrast only a handful of inorganic compounds contain carbon atoms. This acid salt has been used as a capturing agent for CO 2 in situations where such gas. All inorganic acids form hydrogen ions and the conjugate base ions when dissolved in water.
Salts and acids are examples of inorganic compounds called - 26966879 paulis6711 paulis6711 1 week ago SAT High School Salts and acids are examples of inorganic compounds called paulis6711 is waiting for your help. Salts and acids are examples of inorganic compounds called Electrolytes which dissociate in water to release ions. Simple inorganic substances can be grouped as non-metal or metal.
It is used by humans and other mammals to store carbohydrate in the liver. You will soon discover how these two elements combine in the foods you eat in the compounds that make up your body structure and in the chemicals that fuel your functioning. These inorganic acids are either oxygenless or oxoacids.
Click to see full answer. Therefore Salts are not Organic compounds but inorganic compounds with exceptio Organic compounds are sometimes called carbon compounds. A salt is a compound formed when an acid neutralizes a base.
When the atoms are similar the substance is known as a molecule therefore not all molecules are compounds. These compounds are inorganic. Commonly used inorganic acids are sulfuric acid H 2 SO 4 hydrochloric acid HCl and nitric acid HNO 3.
They can be grouped into salts acids and bases. The organic molecule called _____ is formed of branched chains of sugar units. Chlorosulphonic acid HClSO 3 Sulfuric acid H 2 SO 4 Hydrobromic acid HBr Phosphoric acid H 3 PO 4 Fluosulphonic acid HFSO 3 Hydroiodic acid HI Nitric acid HNO 3.
When none of the cations or anions that make up a salt are organic the salt is said to be mineral inorganic. Carbon dioxide CO 2 is one of the few examples. At atom that has gained or lost electrons is called an ion.
They vary from weak to strong acids and also are mono di or even triprotic. Name the class of organic compound that is comprised of subunits consisting of a phosphate group a sugar and a nitrogen-containing base. Also called sodium hydrogen carbonate IV it is a white crystalline solid soluble in.
A great many inorganic compounds do contain hydrogen atoms such as water H 2 O and the hydrochloric acid HCl produced by your stomach. New questions in SAT. These inorganic acids are either oxygenless or oxoacids.
Salts and acids are examples of inorganic compounds called _____ which dissociate in water to release ions. Organic compounds are covered later in the chapter. An inorganic compound is a substance that does not contain both carbon and hydrogen.
Inorganic Acids and Salts Inorganic acids also called mineral acids are acids derived from one or more inorganic compounds. Examples of Mineral acids. Organic compounds are covered later in the chapter.
Inorganic Acids and Salts. Inorganic compounds essential to human functioning include water salts acids and bases. An ionic bond is formed by the transfer of electrons between atoms.
Examples for oxygenless acids are. An organic compound then is a substance that contains both carbon and hydrogen. Add your answer and earn points.
The following section examines the three groups of inorganic compounds essential to life. With reference to the number of hydrogen atoms they are either mono- di- or tribasic. Electrolytes Chemicals called __________ are those that can combine with H when hydrogen ions are in excess and release H when hydrogen ion levels are low.
An example of sedimentation occurs in the blood test that establishes sedimentation rate. Salts and acids are examples of inorganic compounds called _____ which dissociate in water to release ions. Salts and acids are examples of inorganic compounds called.
Examples of acid salts Sodium bicarbonate NaHCO3. The organic molecule called _____ is formed of. This separation of particles from a suspension is called sedimentation.
Water salts acids and bases. The following section examines the three groups of inorganic compounds essential to life. All salts are combinations of cations and anions.
A strong acid reacting with a strong base produces a salt. Water salts acids and bases. Water is a lubricant and cushion a heat sink a component of liquid mixtures a byproduct of dehydration synthesis reactions and a reactant in hydrolysis reactions.
Nucleic acids are composed of building blocks called amino acids. Acids produced by metabolism can potentially cause an _____ in pH of blood. Important in regulation of body temperature.
The symbol Na represents a sodium atom that has lost an electron. Salts and acids are examples of inorganic compounds called _____ which dissociate in water to release ions. This one examples of Inorganic Compounds found at home.
One of easiest acid that can be found at home is Sulphuric Acid H 2 SO 4. Carbon dioxide CO 2 is one of the few examples. The Mineral salts They are a type of inorganic compound.
Related to Differences between Acid Base and Salt 3. An inorganic acid also called a mineral acid is an acid derived from one or more inorganic compounds. Formerly known as vitriol it is used in lead-acid batteries for cars and other vehicles.
With reference to the number of hydrogen atoms they are either mono- di- or tribasic. Carbon compounds are made of carbon bonded to other atoms with exception of carbonates and hydrogen carbonates. Mineral acids are acids derived from inorganic compounds.
A great many inorganic compounds do contain hydrogen atoms such as water H 2 O and the hydrochloric acid HCl produced by your stomach. In contrast only a handful of inorganic compounds contain carbon atoms. The ionic compounds constitute the majority of inorganic compounds meaning that ionic bond is responsible for holding the compound together.
Inorganic acids range from superacids such as perchloric acid HClO 4 to. Glucose and glycogen are examples of what group of organic compounds. That is they do not contain both hydrogen and carbon.
Salts and acids are examples of inorganic compounds called _____ which dissociate in water to release ions. Necessary reactant for energy-releasing metabolic reactions.

Inorganic Compounds Essential To Human Functioning Anatomy And Physiology I

Inorganic Compounds Essential To Human Functioning Anatomy And Physiology I

Difference Between Organic And Inorganic Compounds Key Differences
0 Response to "Salts and Acids Are Examples of Inorganic Compounds Called"
Post a Comment